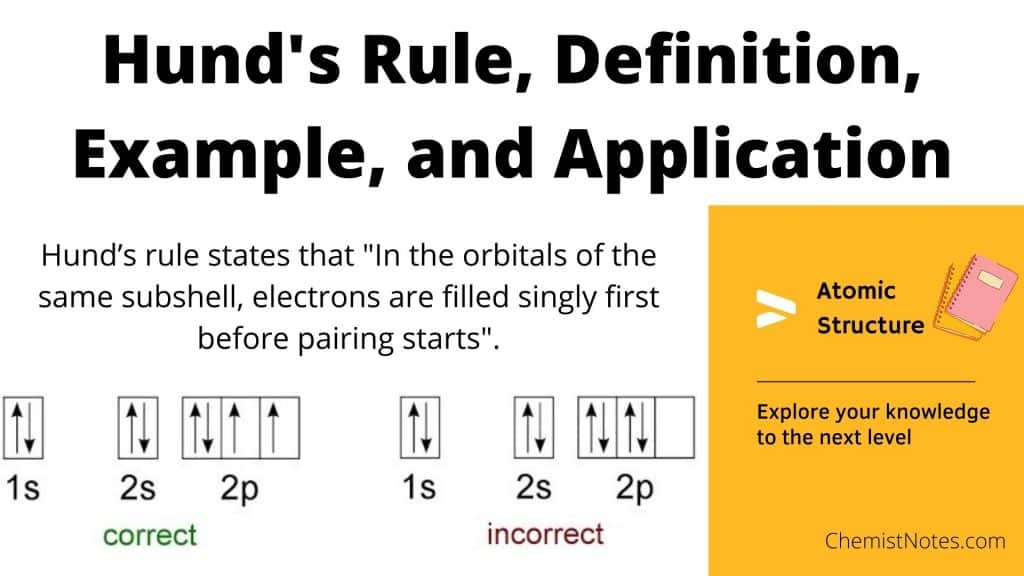
What Is The Difference Between Hund's Rule And Aufbau Principle . Aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the main principles. This chemistry video explains what is the aufbau's principle, hund's rule, and pauli's exclusion. Lower energy orbitals fill before higher energy orbitals. The aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill orbitals, while. Hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each. The aufbau principle dictates the distribution of electrons among the subshells of the atom, and hund’s rule of maximum multiplicity explains. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. One electron goes into each until all of.
from chemistnotes.com
The aufbau principle dictates the distribution of electrons among the subshells of the atom, and hund’s rule of maximum multiplicity explains. Lower energy orbitals fill before higher energy orbitals. Aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the main principles. This chemistry video explains what is the aufbau's principle, hund's rule, and pauli's exclusion. Hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each. The aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill orbitals, while. One electron goes into each until all of. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle.
Hund's Rule, Definition, Example, and Application Chemistry Notes
What Is The Difference Between Hund's Rule And Aufbau Principle Hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. This chemistry video explains what is the aufbau's principle, hund's rule, and pauli's exclusion. Aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the main principles. Lower energy orbitals fill before higher energy orbitals. The aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill orbitals, while. One electron goes into each until all of. Hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each. The aufbau principle dictates the distribution of electrons among the subshells of the atom, and hund’s rule of maximum multiplicity explains.
From www.youtube.com
Electron Configuration (Aufbau principle and Hund's rule) YouTube What Is The Difference Between Hund's Rule And Aufbau Principle Lower energy orbitals fill before higher energy orbitals. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. The aufbau principle dictates the distribution of electrons among the subshells of the atom, and hund’s rule of maximum multiplicity explains. One electron goes into each until all of. This chemistry. What Is The Difference Between Hund's Rule And Aufbau Principle.
From www.youtube.com
The Given Electronic Configuration violates Hund's Rule, Aufbau What Is The Difference Between Hund's Rule And Aufbau Principle One electron goes into each until all of. Lower energy orbitals fill before higher energy orbitals. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. Aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the main principles. This chemistry video. What Is The Difference Between Hund's Rule And Aufbau Principle.
From www.youtube.com
Electrons 2 Electron Configs, Aufbau, Pauli, & Hund's Rules YouTube What Is The Difference Between Hund's Rule And Aufbau Principle This chemistry video explains what is the aufbau's principle, hund's rule, and pauli's exclusion. Hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each. The aufbau principle dictates the distribution of electrons among the subshells of the atom, and hund’s rule of maximum multiplicity. What Is The Difference Between Hund's Rule And Aufbau Principle.
From www.coursehero.com
[Solved] IF U HAVE A DEEPER KNOWLEDGE ABOUT THE AUFBAU PRINCIPLE, PAULI What Is The Difference Between Hund's Rule And Aufbau Principle The aufbau principle dictates the distribution of electrons among the subshells of the atom, and hund’s rule of maximum multiplicity explains. Hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each. Lower energy orbitals fill before higher energy orbitals. This chemistry video explains what. What Is The Difference Between Hund's Rule And Aufbau Principle.
From pediaa.com
Difference Between Aufbau Principle and Hund's Rule Theory What Is The Difference Between Hund's Rule And Aufbau Principle Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. Aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the main principles. This chemistry video explains what is the aufbau's principle, hund's rule, and pauli's exclusion. One electron goes into each. What Is The Difference Between Hund's Rule And Aufbau Principle.
From chemistnotes.com
Hund's Rule, Definition, Example, and Application Chemistry Notes What Is The Difference Between Hund's Rule And Aufbau Principle Hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each. One electron goes into each until all of. The aufbau principle dictates the distribution of electrons among the subshells of the atom, and hund’s rule of maximum multiplicity explains. Aufbau’s principle, hund’s rule, and. What Is The Difference Between Hund's Rule And Aufbau Principle.
From www.reddit.com
Aufbau principle and hund’s rule r/chemistry What Is The Difference Between Hund's Rule And Aufbau Principle Aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the main principles. Lower energy orbitals fill before higher energy orbitals. The aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill orbitals, while. Three rules that help define electron positions. What Is The Difference Between Hund's Rule And Aufbau Principle.
From thechemistrynotes.com
Hund's Rule of Maximum Multiplicity Explanation, Importance, Examples What Is The Difference Between Hund's Rule And Aufbau Principle Aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the main principles. One electron goes into each until all of. Lower energy orbitals fill before higher energy orbitals. This chemistry video explains what is the aufbau's principle, hund's rule, and pauli's exclusion. The aufbau principle and hund’s rule both describe how electrons. What Is The Difference Between Hund's Rule And Aufbau Principle.
From sciencenotes.org
Hund's Rule Definition and Examples What Is The Difference Between Hund's Rule And Aufbau Principle Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. Aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the main principles. Hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied. What Is The Difference Between Hund's Rule And Aufbau Principle.
From mungfali.com
Difference Between Aufbau Principle And Hund's Rule 1C1 What Is The Difference Between Hund's Rule And Aufbau Principle The aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill orbitals, while. Lower energy orbitals fill before higher energy orbitals. One electron goes into each until all of. The aufbau principle dictates the distribution of electrons among the subshells of the atom, and hund’s rule of maximum. What Is The Difference Between Hund's Rule And Aufbau Principle.
From general.chemistrysteps.com
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle What Is The Difference Between Hund's Rule And Aufbau Principle Hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each. This chemistry video explains what is the aufbau's principle, hund's rule, and pauli's exclusion. Aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the main principles.. What Is The Difference Between Hund's Rule And Aufbau Principle.
From general.chemistrysteps.com
Aufbau's Principle, Hund's Rule, and Pauli's Exclusion Principle What Is The Difference Between Hund's Rule And Aufbau Principle Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. The aufbau principle dictates the distribution of electrons among the subshells of the atom, and hund’s rule of maximum multiplicity explains. One electron goes into each until all of. This chemistry video explains what is the aufbau's principle, hund's. What Is The Difference Between Hund's Rule And Aufbau Principle.
From www.chemistrylearner.com
Hund’s Rule Statement, Definition, and Example. What Is The Difference Between Hund's Rule And Aufbau Principle One electron goes into each until all of. Aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the main principles. Hund's rule states that orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron and that each. The aufbau principle and hund’s rule. What Is The Difference Between Hund's Rule And Aufbau Principle.
From thechemistrynotes.com
Aufbau Principle Examples, Limitations and Exception What Is The Difference Between Hund's Rule And Aufbau Principle Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. Lower energy orbitals fill before higher energy orbitals. The aufbau principle dictates the distribution of electrons among the subshells of the atom, and hund’s rule of maximum multiplicity explains. One electron goes into each until all of. Hund's rule. What Is The Difference Between Hund's Rule And Aufbau Principle.
From byjus.com
Difference between Hunds rule and Pauli exclusion principle What Is The Difference Between Hund's Rule And Aufbau Principle The aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill orbitals, while. Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. The aufbau principle dictates the distribution of electrons among the subshells of the atom,. What Is The Difference Between Hund's Rule And Aufbau Principle.
From www.vrogue.co
Difference Between Hunds Rule And Pauli Exclusion Pri vrogue.co What Is The Difference Between Hund's Rule And Aufbau Principle Aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the main principles. The aufbau principle and hund’s rule both describe how electrons fill orbitals, but the aufbau principle explains the order in which electrons fill orbitals, while. Lower energy orbitals fill before higher energy orbitals. The aufbau principle dictates the distribution of. What Is The Difference Between Hund's Rule And Aufbau Principle.
From www.youtube.com
What is the difference between Aufbau principle and Hund's rule? YouTube What Is The Difference Between Hund's Rule And Aufbau Principle Aufbau’s principle, hund’s rule, and pauli’s exclusion principle are rules for writing electron configurations, so go over the main principles. The aufbau principle dictates the distribution of electrons among the subshells of the atom, and hund’s rule of maximum multiplicity explains. One electron goes into each until all of. This chemistry video explains what is the aufbau's principle, hund's rule,. What Is The Difference Between Hund's Rule And Aufbau Principle.
From www.pinterest.co.uk
Aufbau Principle, Pauli Exclusion Principle and Hund's Rule Aufbau What Is The Difference Between Hund's Rule And Aufbau Principle Three rules that help define electron positions within an atom are hund's rule, the pauli exclusion principle, and the aufbau principle. Lower energy orbitals fill before higher energy orbitals. The aufbau principle dictates the distribution of electrons among the subshells of the atom, and hund’s rule of maximum multiplicity explains. The aufbau principle and hund’s rule both describe how electrons. What Is The Difference Between Hund's Rule And Aufbau Principle.